Medical Network April 25th   On April 23, the Drug Evaluation Center of the General Administration of the People's Republic of China issued the “Publicity of the Application for Registration of Drugs to be included in the Priority Review Procedure (Twenty-eighth Batch)â€.

According to the Opinions of the General Administration on Encouraging Drug Innovation to Implement Priority Evaluation and Approval (Food and Drug Administration and Chemicals [2017] No. 126), the Center organizes experts to review and demonstrate the application for drug registration for priority review. The drug registration application for review and its applicant shall be publicized, and the publicity period shall be 5 days. A total of 24
In the Opinions on Encouraging Drug Innovation to Implement Priority Review and Approval issued by the General Administration on December 29, 2017 (Food and Drug Administration Chemicals [2017] No. 126), the scope of priority review and approval is defined as:
è¯å“ Drug registration application with obvious clinical value and one of the following conditions:
1. Application for registration of innovative drugs that are not listed for sale in China or abroad.
2. Transfer to an application for registration of innovative drugs produced in China.
3. Use advanced preparation technology, innovative treatment methods, and drug registration applications with obvious therapeutic advantages.
4. The application for the clinical trial of the drug for 3 years before the expiration of the patent and the application for the production of the drug one year before the expiration of the patent.
5. Applicant's application for new drug clinical trials in the United States and the European Union that are simultaneously applied for and approved for drug clinical trials; drug registration applications that are produced in the same production line in China and are simultaneously applied for listing in the US and EU drug approval agencies and have passed their on-site inspections. .
6. Application for registration of Chinese medicine (including ethnic medicines) with clear clinical positioning in the prevention and treatment of major diseases.
7. It is included in the national science and technology major project, the national key research and development plan, and the application for new drug registration by the National Clinical Medical Research Center for clinical trials and approved by the central management department.
è¯å“ Drug registration application for the prevention and treatment of the following diseases with obvious clinical advantages:
1. AIDS; 2. tuberculosis; 3. viral hepatitis; 4. rare diseases; 5. malignant tumors; 6. children's drugs; 7. elderly patients with multiple and multiple diseases.
â–Other
1. In the quality consistency evaluation of generic drugs, it is necessary to change the supplementary application for re-declaration of the approved process;
2. In the self-inspection and verification project of the “Notice on Carrying out Self-examination and Verification of Drug Clinical Trial Data†(No. 117 of the State Food and Drug Administration announced in 2015), the applicant took the initiative to withdraw and changed the quality of the original drug. A generic drug registration application that is re-declared after the standard is improved;
3. Drug application for clinically urgent and market shortage. The list of specific varieties is proposed by the National Health and Family Planning Commission and the Ministry of Industry and Information Technology. The Drug Evaluation Center of the Food and Drug Administration (hereinafter referred to as the Drug Evaluation Center) organizes relevant departments and experts to demonstrate.
4. In the case of a major threat to public health , the application for registration of a drug that has obtained a compulsory license will be given priority for review and approval. The situation in which public health is seriously threatened and the procedures for initiating compulsory licensing are prescribed by the National Health and Family Planning Commission and relevant departments.
Create a history!
The new drug was applied for two days to complete the technical review. This speed of light review first fell to the Merck's nine-month HPV (human papillomavirus) vaccine.
On April 20, the State Food and Drug Administration just accepted the application for the nine-price cervical cancer vaccine in Merck. On the 23rd and 24th, the two drug numbers completed the technical review.
Some analysts believe that according to the approval speed, it is expected to be formally approved in May this year, and will be officially sold in China at the end of the third quarter or the fourth quarter.
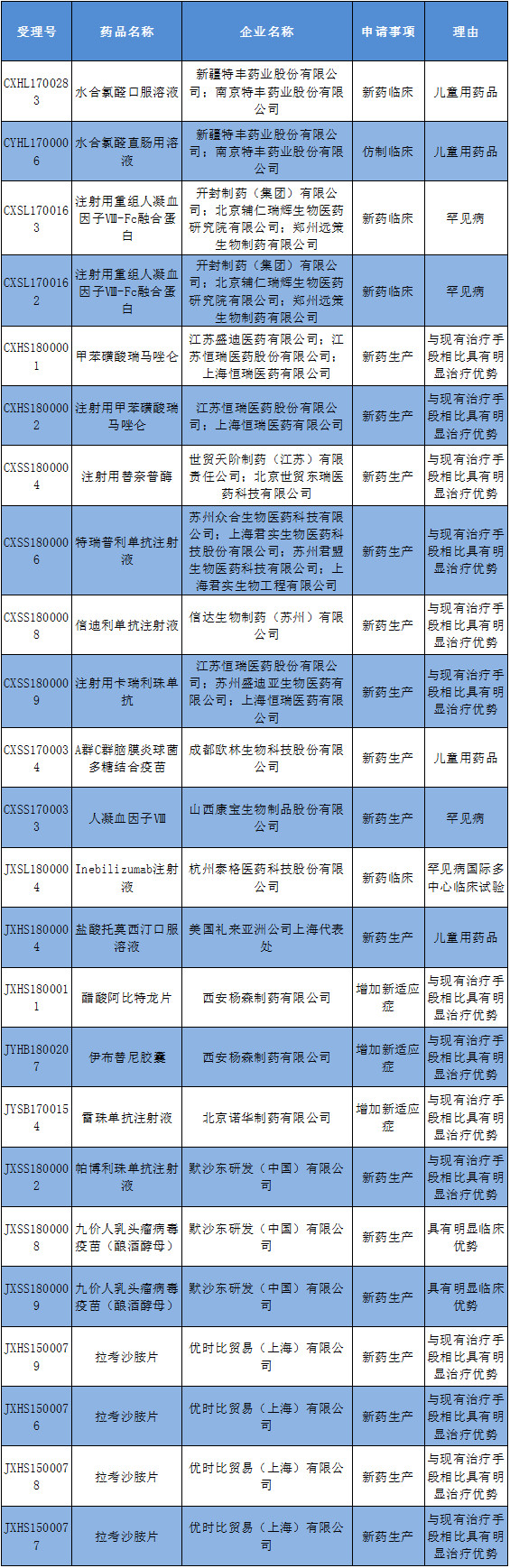
Attachment: List of applications for drug registration approved for inclusion in the priority review process:
Foot Spa Machine,Foot Tub Spa Pedicure,Foot Bath Massager,Bubble Foot Spa
Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootspa.com