Plant heat stress has become a topic of general concern in the scientific community due to the long hot summers and the expected future global warming. Many different types of measurements have been used to study plant heat stress, including NPQ, Fv / Fm, OJIP and quantum photosynthetic yield Y (II) chlorophyll fluorescence measurements. This application note discusses which protocols are the most efficient, fastest, and easiest to measure.
NPQ:
Although NPQ can be used to measure heat stress (Schreiber U.2004), (Tang Y., Wen X., Lu Q. Yang Z., Cheng Z., & Lu C. 2007) (Haldiman P, & Feller U. 2004), this It is a time consuming measurement and needs to be very detailed. The recommended darkness in the field is 8 to 12 hours, or overnight. The dark adaptation time recommended in the laboratory ranges from 12 to 24 hours. (Maxwell and Johnson 2000). In the field, NPQ does not completely relax overnight, as recovery from photoinhibition takes up to sixty hours (Lichtenthaler 2004). Therefore, there are always some residual NPQs that are not measured and accepted. The standard used to measure NPQ is the height of Fm in Fv / Fm (Baker 2008). Therefore, it is extremely important to compare only samples having the same or very similar Fv / Fm values. Otherwise, it will be compared to measurements with different non-standard size scales. In addition to long dark adaptation times, NPQ must be measured after the blade has reached steady-state photosynthesis. Steady-state photosynthesis usually uses artificial light sources, which require 15 to 20 minutes at stable light levels (Maxwell and Johnson) 2000). NPQ can detect heat stress at about 35 ° C and higher (Haldiman P, & Feller U. 2004). The NPQ saturation pulse map is a good way to study the recovery of plants from heat stress, as NPQ decreases over time and quantum photosynthetic yield increases (Schreiber U.2004).
Fv/Fm and OJIP:
     Fv/Fm and OJIP can be measured more quickly by dark adaptation; however, studies have shown that their value for heat stress measurements is very limited. Dark adaptation time usually ranges from twenty minutes to overnight or before dawn. A survey of published literature indicates that Fv / Fm can only detect heat stress at about 45 ° C (Haldiman P, & Feller U. 2004), (Crafts-Brander and Law 2000), OJIP can only detect about 44 ° C Or higher temperature heat stress (Strasser 2004).
Quantum photosynthetic yield (â–³F/Fm'):
Finally, perhaps the best option is to use quantum photosynthetic yield (ΔF / Fm') to detect and measure heat stress. ΔF / Fm' is a light-adaptive measurement performed at steady-state photosynthesis or at light levels that have not changed for 15 to 20 minutes (Maxwell and Johnson 2000). However, since most researchers use ambient light, The blades are usually in a stable state without additional waiting. The measurement takes only two to five seconds, and a large number of leaves can be tested in a short time. ΔF / Fm' changes with the light level, so the sample to be controlled is controlled. It is important to measure the light level, or to measure light levels with PAR leaf clips, and to compare samples with similar light levels. Cloudy weather may cause problems in field measurements because steady-state photosynthesis does not always In addition, it is common to measure the leaves at the top of the canopy in the field because sun spots and vegetation movements caused by the wind prevent the leaves from reaching steady state photosynthesis. The literature shows that ΔF / Fm' is about 35 ° C or higher. Temperature detection of heat stress (Haldiman P, & Feller U. 2004), (Dascaliuc A., Ralea t., Cuza P. 2007). This measurement provides NPQ to heat in seconds. Forced sensitivity.
△F / Fm' can be used for more advanced chlorophyll fluorescence instruments, such as Opti-Sciences models; OS5p+ and OS1p. NPQ is best handled by OS5p because it has a very stable actinic light source to drive photosynthesis. For comparison, it was found that CO2 gas exchange can detect and measure heat stress at about 30 °C (Haldiman P, & Feller U. 2004). For those wishing to use both gas exchange and chlorophyll fluorescence, the combination of fluorescence and CO2 gas exchange can be performed using OS5p in conjunction with the ADC LCpro + CO2 gas exchange system.
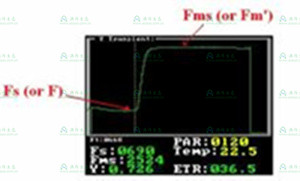
♦ Quantum photosynthetic yield - what is its significance and its limitations
♦ Dark adaptation - how long is long enough
♦ PAR measurement and ETR - how does it mean and its limitations
♦ Quenching measurement - what it means, current measurement assessment and limitations
♦ Early water stress measurement
♦ Nitrogen stress measurement
♦ How state transition affects fluorescence measurement
♦ Fast light curve - how to use it and its limitations
♦ Light curve - how it is used and what it means.
references:
Baker NR, (2008) “Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo†Annu. Rev. Plant Biol. 2008. 59:89–113
Crafts-Brandner SJ, Law RD (2000) Effects of heat stress on the inhibition and recovery of ribulase-1, 5-biphsphate carboxylase/ oxygenase activation state. Planta (2000) 212: 67-74
Dascaliuc A., Ralea t., Cuza P., (2007) Influence of heat shock on chlorophyll fluorescence of white oak
(Quercus pubescens Willd.) leaves. Photosynthetica 45 (3): 469-471, 2007
Haldimann P, & Feller U. (2004) Inhibition of photosynthesis by high temperature in oak
(Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat
Dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase.
Lichtenthaler HK, Burkart S., (1999) Photosynthesis and high light stress. Bulg. J. Plant Physiol., 1999, 25(3-4), 3-16
Lichtenthaler HK, Babani F. (2004) Light Adaption and Senescence of the Photosynthetic Apparatus.
Changes in Pigment Composition, Chlorophyll Fluorescence Parameters and Photosynthetic Activity. From Chapter 28, "Chlorophyll a Fluorescence a Signature of Photosynthesis", edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 716
Maxwell K., Johnson G. N, (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental
Botany Vol. 51, No. 345, pp. 659-668- April 2000
Schreiber U, (2004) Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An
Overview From Chapter 11, "Chlorophyll a Fluorescence a Signature of Photosynthesis", edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 279-319
Strasser RJ, Tsimilli-Michael M., and Srivastava A. (2004) - Analysis of Chlorophyll a Fluorescence
Transient. From Chapter 12, “Chlorophyll a Fluorescence a Signature of Photosynthesisâ€, edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 340
Tang Y., Wen X., Lu Q., Yang Z., Cheng Z., Lu C., (2007) Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiology, Feb. 2007 Vol. 143, pp629-638
YT-H711
YT-H711
Shenzhen Sunshine Technology Co.,Ltd , https://www.szyatwin.com