Cingulate announces new trimodal oral formulation test data for ADHD treatment
January 18, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];On January 15th, Cingulate Therapeutics, a biopharmaceutical company focused on developing innovative products using precision timed release platform technology, said that the company's article titled "New Trimodal Oral Formulation CTX-1301 in Healthy Adults" Posters on drug efficacy and pharmacokinetic analysis of methyl ester release were published at the 2018 American Association of ADHD and Related Diseases (APSARD). This poster marks the final release of CTX-1301 pharmacokinetics and drug delivery mechanism research evaluation data. CTX-1301 is the company's patented first-line treatment for attention deficit hyperactivity disorder (ADHD), which utilizes an innovative patented erosion barrier technology developed by Cingulate in collaboration with Bio-Images Drug Delivery. OralogiKTM.

The study consisted of three treatment arms: 1, Focalin® XR (Lipperate Hydrochloride Controlled Release Capsule); 2. CTX-1301 with radiolabeled second layer; 3. CTX with radiolabeled third layer 1301. Drug angiography monitors the transport of radiolabeled tablets in the gastrointestinal tract. Importantly, the mean time to final release of CTX-1301 was 10.3 hours, compared to the Focalin XR group (17.05 ng-hr/mL), the average AUC of 8-24 hours after administration of the two CTX-1301 treatment groups had A statistically significant difference (p < 0.005) confirmed higher drug plasma levels of CTX-1301. Compared to Focalin XR, CTX-1301 also provided a smoother, controlled d-MPH decline after the last dose.
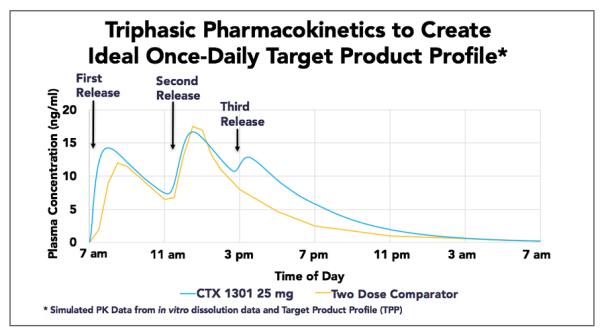
The researchers found that CTX-1301 provides stable trimodal release of d-MPH hydrochloride compared to the controlled release formulation Focalin® XR with bimodally released d-MPH properties. 4 hours of extended d-MPH plasma levels.
Dr. Shane J. Schaffer, CEO and President of Cingulate, said: “These results are a major achievement. Before the company's necessary research has been approved by the FDA, we have learned how candidate products work in the human body. The results show that Our platform is capable of providing precise, timed doses for a variety of therapeutics, and we are currently preparing a new drug clinical application (IND) for innovative ADHD formulations."
Dr. Raul R. Silva, co-founder and chief scientific officer of the company, said: "Doping is the most commonly used drug for the treatment of ADHD in the present and for the foreseeable future. Although research and clinical results support their continued use, there is currently no one. The product provides the target drug after a single dose to meet all the needs of patients with ADHD. The results of this trial show that the precise timed release (PTRTM) technology can confer clinically desirable product characteristics for CTX-1301 and CTX-1302. To allow patients to achieve a better prognosis and quality of life. At the same time, future trials will include studies in simulated classrooms and workplaces that correlate the pharmacokinetics and clinical efficacy of the two study drugs."
Cingulate's innovative precision timed release platform opens the door to the development and production of multiple doses of daily dosing. The company initially focused on the development of therapeutic drugs for attention deficit/hyperactivity disorder. The company's two drug candidates, CTX-1301 (dextramethylphenidate) and CTX-1302 (dextroamphetamine), are used to treat ADHD in all patients (children, adolescents, and adults) using an innovation The flexible core production technology enables rapid onset of action and lasts throughout the day, while providing drug release characteristics with controlled plasma drug levels to optimize treatment options. The company has completed a clinical validation phase 1 trial and plans to launch a comprehensive clinical program for both drugs in 2018. The company expects that the IND for drug submissions in the second quarter of 2018 will be approved through accelerated 505(B)(2) approval. (Sina Pharmaceutical Compilation / David)
Article Reference Source: Cingulate Therapeutics Presents New Data from its First in Human Proof of Concept Trial of CTX-1301 at the 2018 APSARD Annual Meeting
Vitamin A in fresh corn also has a certain auxiliary effect on the prevention and treatment of common dry eye, bronchitis, dry skin and nerve paralysis. Fresh corn is rich in lysine (very little in dry corn), which is an essential nutrient for the human body. Studies have found that eating more fresh corn can also inhibit the side effects of anticancer drugs on the human body. The cellulose in fresh corn is more and longer, and its amount is 6 to 8 times that of polished rice and flour. Fiber can make the stool smooth, so eating corn often can prevent constipation and hemorrhoids, and can also reduce the occurrence of gastrointestinal diseases. At the same time, it is also beneficial for preventing rectal cancer, arteriosclerosis, and lowering cholesterol. In addition, fresh corn is a bit hard and needs to be chewed hard when eating, which can exercise the teeth and promote saliva production and strong gums.
Waxy Corn,Yellow Waxy Corn,Glutinous Corn Cob,Yellow Glutinous Corn Cob
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com